Introduction:
Dupilumab is a human monoclonal antibody that inhibits signaling of IL-4 and IL-13 and is currently approved by the Food and Drug Administration for the treatment of atopic dermatitis (AD). While causality has not been established, sporadic reports have emerged associating dupilumab use with the onset, progression, or subsequent diagnosis of cutaneous T-cell lymphomas, in particular mycoses fungoides (MF). The natural history of MF diagnosed after dupilumab exposure remains unclear. We performed a retrospective matched cohort study to compare the clinical characteristics and treatment outcomes of patients with MF based on prior dupilumab exposure.
Methods:
Through the Memorial Sloan Kettering (MSK) Research & Technology data delivery platform (DataLine), we conducted a search for any patient seen by a provider in the MSK Cutaneous Lymphoma Clinic whose electronic record contained the word ‘dupilumab’ or ‘Dupixent.‘ Once the initial query was performed, we individually reviewed each patient to confirm: (1) A histologically-confirmed diagnosis of MF by an MSK dermatopathologist, and (2) Use of dupilumab prior to the histological diagnosis of MF. After identifying a cohort of patients with confirmed MF after dupilumab use (cases), we identified a matched cohort of patients with confirmed MF and no prior dupilumab use (controls). Specifically, we matched patients for stage at diagnosis, date of birth and age at diagnosis (+/- 5 years), and date of presentation (+/- 5 years). Clinical data and treatment outcomes were collected by clinical review. Baseline characteristics were summarized using descriptive statistics and compared between the two groups using the Wilcoxon rank sum test, Pearson's Chi-squared test, and Fisher's exact test. Overall survival (OS), progression-free survival (PFS), and recurrence-free survival (RFS) were estimated as the elapsed time from initial diagnosis to death, disease recurrence, and progression, respectively. Kaplan-Meier estimates were stratified by exposure to dupilumab, and differences between the estimates were compared using the log-rank test.
Results:
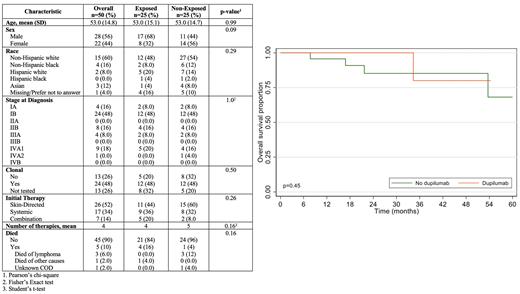
We identified 25 patients who presented with a new, histologically-confirmed diagnosis of MF following dupilumab treatment for a diagnosis of AD. In 16 of these patients, AD was diagnosed based on a skin biopsy, whereas in 9 patients the diagnosis was based on clinical features only. Among 16 patients with known duration of dupilumab use, the median duration of use was 5.0 months (min/max: 1.5-24 months). In total, 14 patients were diagnosed with early-stage disease (IA n=2, IB n=12) and 11 were diagnosed with advanced stage disease (IIB n=4, IIIA n=2, IVA1 n=5). The estimated time from dupilumab treatment to lymphoma diagnosis was 10.2 months, and the median age at lymphoma diagnosis was 53 years (min/max: 28-84). Compared to the cohort of 25 matched patients with MF and no prior dupilumab use ( Table 1), no difference was observed in race, sex, initial therapy (skin-directed vs. systemic), or total number of therapies. The median follow-up time for patients exposed to dupilumab was 13.2 months (95% CI: 6.8-21.9), and 20.7 months (95% CI: 13.4-27.8) for those not exposed. No differences were observed in OS, PFS, or RFS between the exposed (dupilumab) and non-exposed (non-dupilumab) cohorts ( Figure 1). Two-year OS, PFS, and RFS in the non-dupilumab cohort was 85.0%, 47.3%, and 70.7%, compared to 100.0%, 41.5%, and 79.6% in the dupilumab cohort. One patient in the dupilumab-exposed cohort died of a non-lymphoma malignancy, whereas 4 patients in the non-exposed cohort died (3 due to lymphoma, 1 due to unknown causes).
Conclusions:
In summary, we identified a relatively large cohort of patients with MF diagnosed after dupilumab treatment. Patients presented with early and advanced-stage disease. Outcome were comparable to a matched cohort of non-exposed patients with MF. The causal relationship between dupilumab and MF remains unproven and deserves dedicated biological study.
Disclosures
Epstein-Peterson:WebMD: Honoraria; OncLive: Honoraria; Amgen: Research Funding; Viracta: Research Funding; Kymera: Research Funding. Ghione:AstraZeneca Pharmaceuticals: Consultancy; Kyowa Hakko Kirin: Consultancy; Secura Bio: Consultancy; Kite, A Gilead Company: Research Funding. Johnson:Myeloid Therapeutics: Consultancy. Moskowitz:Bristol-Myers Squibb: Research Funding; ADC Therapeutics: Research Funding; Beigene: Research Funding; Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Incyte: Research Funding. Horwitz:Affimed: Research Funding; Shoreline Biosciences, Inc.: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; ADC Therapeutics: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Trillium Therapeutics: Consultancy, Research Funding; Yingli Pharma Limited: Consultancy; Tubulis: Consultancy; Verastem/SecuraBio: Research Funding; Auxilius Pharma: Consultancy; Cimieo Therapeutics: Consultancy; SecuraBio: Consultancy; Abcuro Inc.: Consultancy; Celgene: Research Funding; Takeda: Consultancy, Research Funding; Crispr Therapeutics: Research Funding; Millenium: Research Funding; Seattle Genetics: Research Funding; ONO Pharmaceuticals: Consultancy. Geller:Takeda Pharmaceuticals: Other: Professional services and activities; RAFA Laboratories Ltd: Other: Professional services and activities; UptoDate: Other: Intellectual Property Rights.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal